Differential Scanning Calorimetry & Thermo-Gravimetric Analysis

The Perkin Elmer DSC7
Differential Scanning Calorimetry (DSC) is widely used to characterise the thermophysical properties of polymers. DSC can measure important thermoplastic properties including:
- Melting temperature
- Heat of melting
- Percent crystallinity
- Tg or softening
- Crystallization
- Presence of recyclates/regrinds
- Plasticizers
- Polymer blends (presence, composition and compatibility)
Differential scanning calorimetry or DSC is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference are measured as a function of temperature. Both the sample and reference are maintained at very nearly the same temperature throughout the experiment.
Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned.
The basic principle underlying this technique is that, when the sample undergoes a physical transformation such as phase transitions, more (or less) heat will need to flow to it than the reference to maintain both at the same temperature.
Whether more or less heat must flow to the sample depends on whether the process is exothermic or endothermic. For example, as a solid sample melts to a liquid it will require more heat flowing to the sample to increase its temperature at the same rate as the reference.
This is due to the absorption of heat by the sample as it undergoes the endothermic phase transition from solid to liquid. Likewise, as the sample undergoes exothermic processes (such as crystallization) less heat is required to raise the sample temperature. By observing the difference in heat flow between the sample and reference, differential scanning calorimeters are able to measure the amount of heat absorbed or released during such transitions.
DSC may also be used to observe more subtle phase changes, such as glass transitions. DSC is widely used in industrial settings as a quality control instrument due to its applicability in evaluating sample purity and for studying polymer curing. The result of a DSC experiment is a heating or cooling curve.
This curve can be used to calculate enthalpies of transitions. This is done by integrating the peak corresponding to a given transition. It can be shown that the enthalpy of transition can be expressed using the following equation:
ΔH = KA
where ΔH is the enthalpy of transition, K is the calorimetric constant, and A is the area under the curve. The calometric constant will vary from instrument to instrument, and can be determined by analyzing a well-characterised sample with known enthalpies of transition.
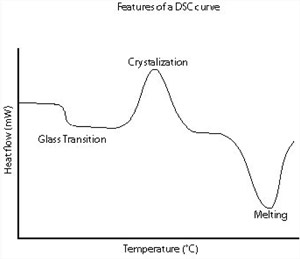
A schematic DSC curve demonstrating the appearance of several common features.
Differential scanning calorimetry can be used to measure a number of characteristic properties of a sample. Using this technique it is possible to observe fusion and crystallization events as well as glass transition temperatures (Tg). DSC can also be used to study oxidation, as well as other chemical reactions.
Glass transitions may occur as the temperature of an amorphous solid is increased. These transitions appear as a step in the baseline of the recorded DSC signal. This is due to the sample undergoing a change in heat capacity; no formal phase change occurs. As the temperature increases, an amorphous solid will become less viscous.
At some point the molecules may obtain enough freedom of motion to spontaneously arrange themselves into a crystalline form. This is known as the crystallization temperature (Tc). This transition from amorphous solid to crystalline solid is an exothermic process, and results in a peak in the DSC signal.
As the temperature increases the sample eventually reaches its melting temperature (Tm). The melting process results in an endothermic peak in the DSC curve. The ability to determine transition temperatures and enthalpies makes DSC an invaluable tool in producing phase diagrams for various chemical systems.
Using differential scanning calorimetry to study the oxidative stability of samples generally requires an airtight sample chamber. Usually, such tests are done isothermally (at constant temperature) by changing the atmosphere of the sample. First, the sample is brought to the desired test temperature under an inert atmosphere, usually nitrogen.
Then, oxygen is added to the system. Any oxidation that occurs is observed as a deviation in the baseline. Such analyses can be used to determine the stability and optimum storage conditions for a compound.
DSC is widely used in the pharmaceutical and polymer industries. For the polymer chemist, DSC is a handy tool for studying curing processes, which allows the fine tuning of polymer properties. The cross-linking of polymer molecules that occurs in the curing process is exothermic, resulting in a positive peak in the DSC curve that usually appears soon after the glass transition.
In the pharmaceutical industry it is necessary to have well-characterised drug compounds in order to define processing parameters. For instance, if it is necessary to deliver a drug in the amorphous form, it is desirable to process the drug at temperatures below those at which crystallization can occur.
In food science research, DSC is used in conjunction with other thermal analytical techniques to determine water dynamics. Changes in water distribution may be correlated with changes in texture. Similar to material science studies, the effects of curing on confectionery products can also be analyzed.
DSC curves may also be used to evaluate drug and polymer purities. This is possible since the temperature range over which a mixture of compounds melts is dependent on their relative amounts.
This effect is due to a phenomenon known as freezing point depression, which occurs when a foreign solute is added to a solution. Consequently, less pure compounds will exhibit a broadened melting peak that begins at lower temperature than a pure compound.
Thermo-Gravimetric Analysis or TGA
Thermogravimetric analysis (TGA) is one of the members of the family of thermal analysis techniques used to characterise a wide variety of materials.
TGA provides complimentary and supplementary characterisation information to the most commonly used thermal technique, DSC.
TGA measures the amount and rate (velocity) of change in the mass of a sample as a function of temperature or time in a controlled atmosphere. The measurements are used primarily to determine the thermal and/or oxidative stabilities of materials as well as their compositional properties.
The technique can analyze materials that exhibit either mass loss or gain due to decomposition, oxidation or loss of volatiles (such as moisture). It is especially useful for the study of polymeric materials, including thermoplastics, thermosets, elastomers, composites, films, fibres, coatings and paints.
TGA measurements provide valuable information that can be used to select materials for certain end-use applications, predict product performance and improve product quality. The technique is particularly useful for the following types of measurements:

The Perkin Elmer TGA7
- Compositional analysis of multi-component materials or blends
- Thermal stabilities
- Oxidative stabilities
- Estimation of product lifetimes
- Decomposition kinetics
- Effects of reactive atmospheres on materials
- Filler content of materials
- Moisture and volatiles content
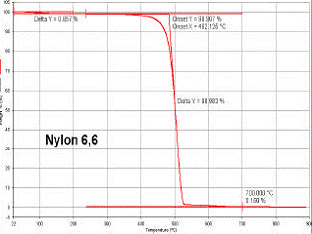
Typical TGA results showing thermal degradation in a Nylon 6,6 product
Telephone:
01299 251 914
Our postal address:
Fleming
Polymer Testing Ltd,
Holly House
Hartlebury
Kidderminster
DY11 7TE UK

